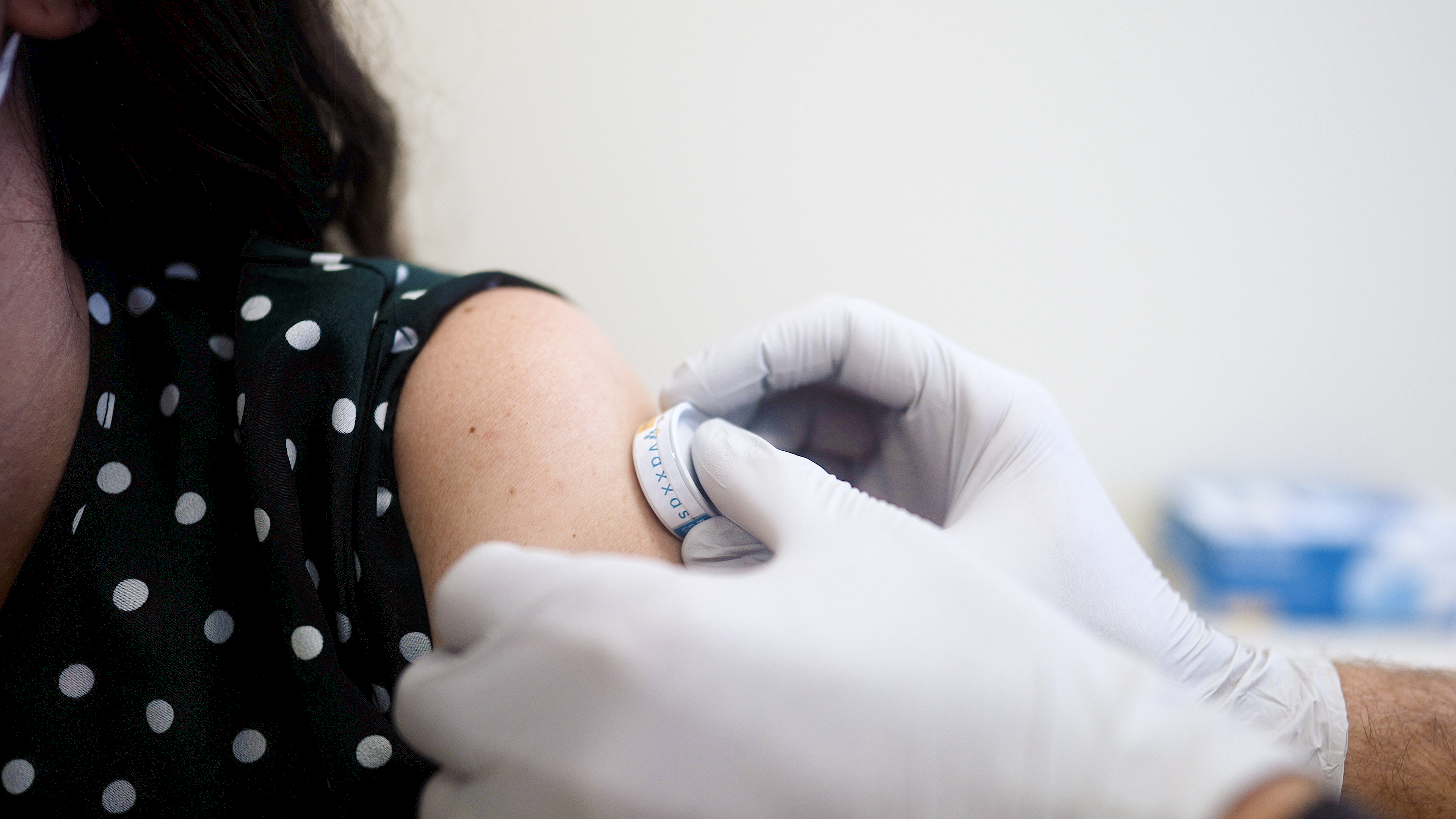
The University of the Sunshine Coast is about to begin a clinical trial to assess a needle-free technology for vaccinating against influenza, in Brisbane, Morayfield and on the Sunshine Coast.
UniSC Clinical Trials has partnered with Australian biotechnology company Vaxxas, developers of the high-density microarray patch (HD-MAP), after running a similar study in 2021 for measles and rubella and currently for COVID-19.
Dr Angus Forster, Chief Technology Officer at Vaxxas, said the study would investigate the safety and tolerability of an influenza vaccine delivered using the company’s HD-MAP technology and assess whether it provides an immune response comparable to a standard intramuscular dose delivered by needle and syringe.
“We are pleased to be partnering again with the team at UniSC Clinical Trials to undertake a Phase One study of an influenza vaccine using our HD-MAP technology,” Dr Forster said.
“We hope to demonstrate that delivering an influenza vaccine using our HD-MAP technology can potentially be just as effective as the traditional intramuscular vaccine needle injection.”
The trial will be led by Dr Nischal Sahai at the UniSC Clinical Trials clinic at South Bank, Brisbane.
“It’s very exciting to be working with innovative Australian vaccination technology that could potentially change the way vaccines are administered around the world,” Dr Sahai said.
“HD-MAP technology could mean that vaccines no longer need to be refrigerated, making it easier to transport them safely to people in remote locations.
“We received a good response from the community for our trial last year with the HD-MAP technology. We hope they will be as excited as we are to participate in this next clinical study,” he said.
The study requires healthy volunteers aged 18-50 years old who are in good general health and have a body mass index within the range of 18-32.
Participants will be required to visit the UniSC Clinical Trials clinics at South Bank in Brisbane or Sippy Downs on the Sunshine Coast approximately six times over a two-and-a-half-month period.
Those interested in participating can find more information at www.usc.edu.au/trials
Related articles

Clinical trial of asthma injection that aims to reduce airway inflammation
9 Oct 2024UniSC Clinical Trials is investigating an injectable antibody therapy designed to reduce inflammation that causes asthma

Trial of disease-modifying treatment for early stages of Alzheimer’s disease
3 Oct 2024A potential new treatment that could improve cognition and memory in patients with mild to moderate dementia due to Alzheimer’s disease is being investigated by the University of Sunshine Coast

Focus on healthier people and planet proves winning formula for UniSC
30 Aug 2024From a ‘game-changing’ koala chlamydia vaccine to a trial of a needle-free flu vaccine, the University of the Sunshine Coast’s success in research and bioinnovation has been recognised in the Life Sciences Queensland GENE Awards
Media enquiries: Please contact the Media Team media@usc.edu.au